South Korea is battling Japan again, this time on the global market to treat gastroesophageal reflux disease (GERD).
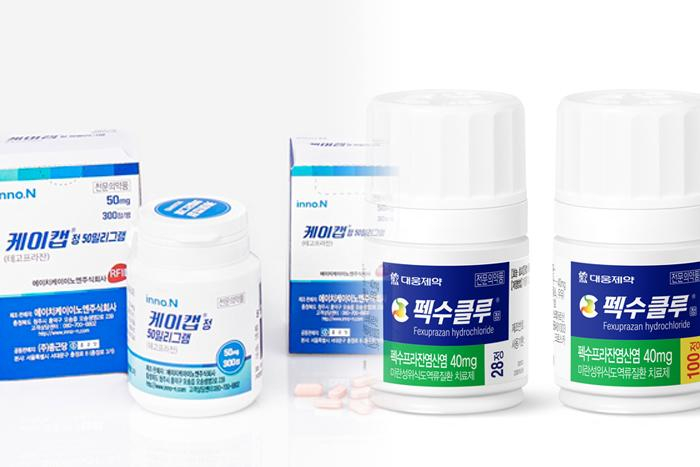
South Korea's pharmaceutical product maker HK Inno.N is expanding the number of licensing offices for its GERD drug K-Cab, and Daewoong Pharmaceutical is doing the same for its
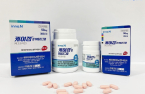
Fexuclu medicine to take on the global market leader Taekcab made by Takeda Pharmaceutical of Japan.
HK Inno.N on Thursday said it recently signed a contract with Europharma, a Brazilian pharmaceutical corporation, to export K-Cab technology. The former will transfer manufacturing technology and know-how to the latter, and Europharma will oversees product development and sales in Brazil for 10 years.
Releasing the value of an export contract for pharmaceutical technology is standard, but not for this deal. Europharma requested that such information remain confidential because doing so will help the company gain a competitive edge against Brazilian market leader Takecab.
Through its technology transfer deal in Brazil, HK Inno.N has laid the foundation for supplying K-Cab throughout the Americas. The Brazilian market for peptic ulcer medicine in 2020 was worth 800 billion won ($651 million), sixth in the world and No. 1 in South America.
HK Inno.N first launched K-Cab in China and the Philippines and seeks to export the drug to 100 countries by 2028.
On the same day, Daewoong Pharmaceutical said it applied for approval of Fexuclu with the Food and Drug Authority of Saudi Arabia, the 11th country where the company has filed such an application under its goal to sell the drug in 20 countries by 2025.
In 2021, the peptic ulcer medicine market in Saudi Arabia was worth 410 billion won, ranking 12th worldwide and No. 1 in the Middle East.
Write to Ji-Hyun Lee at
bluesky@hankyung.com