South Korean medical AI company Lunit said on Monday that its 3D breast tomosynthesis artificial intelligence (AI) image analysis solution, "Lunit Insight DBT", has received Medical Device Directive (MDD) CE certification in the European Union (EU).
The company plans to start selling the device in Europe by the end of this month. The EU's MDR CE certification enhances the performance and quality standards of medical devices compared to the previous Medical Device Directive CE making it essential for companies seeking to enter the European market as the sale of uncertified products will be prohibited from May 2024.
Lunit had previously acquired MDR CE certification for two other products, the chest X-ray AI image analysis solution "Lunit Insight CXR" and the mammography AI image analysis solution "Lunit Insight MMG."
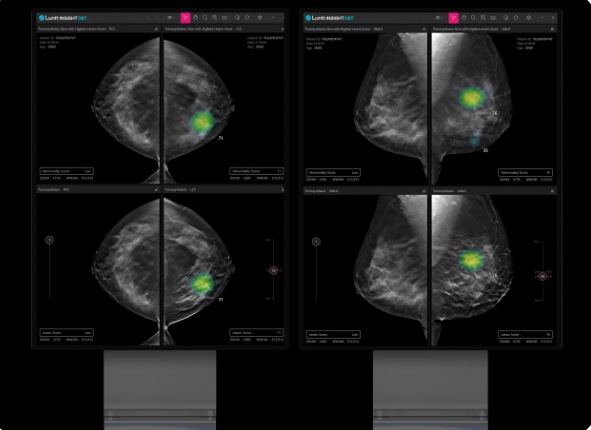
Lunit Insight DBT analyzes 3D breast tomosynthesis images quickly and accurately, assisting healthcare professionals in diagnosing breast cancer. The company developed Lunit Insight DBT based on Lunit Insight MMG. 3D DBT allows for more sophisticated testing than conventional 2D breast imaging and is in high demand in advanced medical institutions in countries such as the US and Europe.
At the "European Congress of Radiology 2023" held in Vienna, Austria, earlier this month, the company presented a research abstract related to Lunit Insight DBT. The company analyzed the accuracy and performance of breast cancer detection for 162 women with an average age of 52 who underwent examination at domestic advanced medical institutions.
The results showed that Lunit Insight DBT demonstrated accuracy comparable to that of experienced radiologists with more than ten years of experience.
"We are ready to sell Lunit Insight DBT in Europe, and we plan to expand sales to other global markets, including the US and Australia, which comply with European regulations," said Lunit CEO Suh Beom-seok.
Lunit also plans to pursue approval procedures for Lunit Insight DBT in the US, the world's largest medical device market, within this year.
Write to Ji-Hyun Lee at
bluesky@hankyung.com