South Korean medical AI company Lunit announced on Tuesday that more than 2,000 medical institutions worldwide have adopted its AI-based diagnostic solution called Lunit Insight.
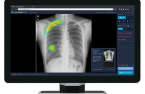
Lunit launched its chest X-ray image analysis solution,
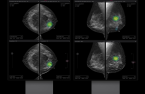
Lunit Insight CXR, and breast imaging analysis solution,
Lunit Insight MMG, in April 2019. Last year in October, it exceeded 1,000 medical institutions that introduced the solution, three years and six months after its launch. After that, the number of customers doubled in about five months, exceeding 2,000.
Among all medical institutions that have introduced Lunit Insight, 84% are overseas customers. The number of overseas customers that introduced the solution more than doubled from 770 in October last year to 1,680 as of March 31 this year. During the same period, the number of domestic institutions introducing the solution increased by about 40%, from 230 to 320.
As the number of institutions adopting the solution increases, revenue has also increased significantly. Lunit's revenue has been more than doubling each year from 1.4 billion won ($1.1 million) in 2020 to 6.6 billion won ($5.1 million) in 2021, and 13.9 billion won ($10.6 million) in 2022.
"After obtaining approval from global regulatory agencies such as those in North America, Taiwan and Europe, the market for the Lunit Insight product line has expanded rapidly," said Lunit CEO Suh Beom-seok. "The usage rate of Lunit products among medical institutions that have adopted AI related to X-rays and breast imaging is estimated to be over 80%, so we expect our market share to expand further in the future."
Write to Jeong Min Nam at
peux@hankyung.com