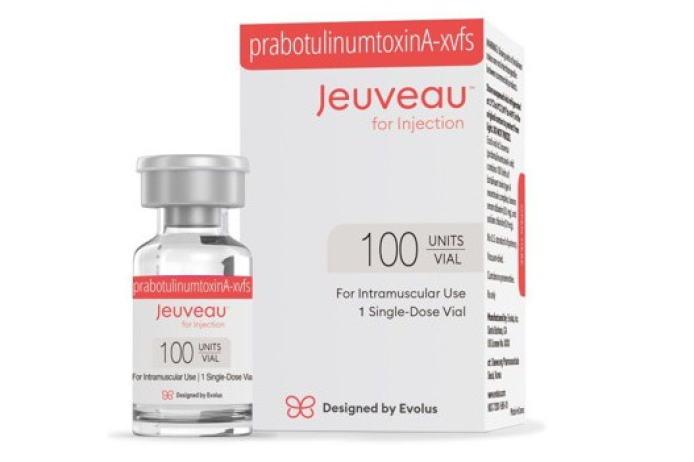
South Korea's Daewoong Pharmaceutical said on Thursday that its local loyalty program for Nabota (American name: Jeuveau) in the US, a botulinum toxin developed by the company, has surpassed 600,000 members.
The program, called Evolus Rewards, offers appointment reservations and benefits for Nabota treatments.
According to Evolus, the US partner of Daewoong Pharmaceutical, a total of 600,000 customers have joined the program since Nabota was launched in the US market in 2020. The number of treatments received through the program has exceeded 1 million cumulatively.
Nearly all members who have undergone the first treatment have registered for the second treatment, with a re-treatment rate of 96%. This is a high re-treatment rate for a loyalty program, and it suggests that Nabota is a product that customers are satisfied with.
The majority of the members are millennials or younger generations. Nabota has achieved an average annual sales growth rate of 62% in the US in the past two years, and its market share has also exceeded 10%.
Write to Ji-Hyun Lee at
bluesky@hankyung.com