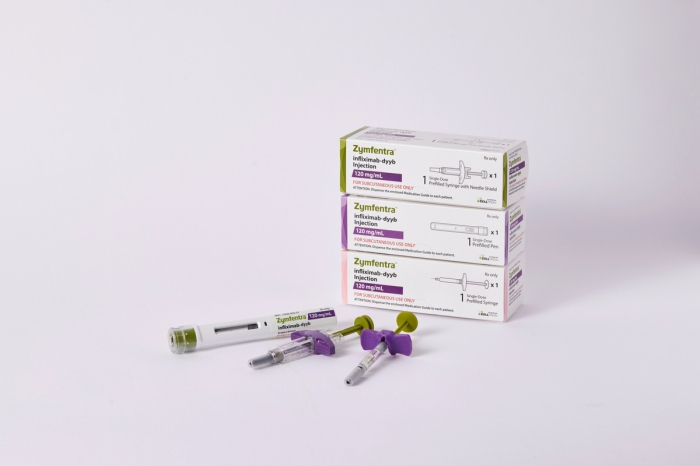
Celltrion Healthcare Co. announced on Thursday that its autoimmune disease treatment Zymfentra (the US product name for Remsima SC) is set to be launched in the United States on Feb. 29 next year.
Remsima SC is a subcutaneous (SC) formulation of the autoimmune disease treatment Remsima, containing the ingredient infliximab, and obtained new drug approval from the US Food and Drug Administration (FDA) last month.
In efforts to establish Zymfentra in the local market, Celltrion Healthcare has provided clinical trial data to multiple pharmacy benefit managers (PBMs) and is currently negotiating for preferred drug inclusion.
PBMs are companies specializing in managing prescription drug services in the United States, and inclusion in the list of drugs managed by PBMs implies entry into the US medical insurance coverage system.
Celltrion Healthcare is actively discussing inclusion with some PBMs, anticipating that inclusion may be possible before or after the launch of Zymfentra.
The company also plans to expand its local workforce, doubling the sales personnel responsible for selling the drug by Jan. next year and increasing promotional and marketing staff by more than three times.
Additionally, it aims to promote Zymfentra at conferences targeting US healthcare professionals and collaborate with major patient advocacy groups to support patients prescribed with the drug.
"As Zymfentra is a key blockbuster product driving Celltrion's integrated 2030 sales target of 12 trillion won ($9.3 billion), we will do our best to actively promote the unique strengths that Zymfentra possesses, ensuring the expansion of prescriptions in the US," said a Celltrion Healthcare official.
Write to Dae-Kyu Ahn at
powerzanic@hankyung.com