South Korea's bio-regenerative medical firm CGBio announced on Tuesday that its Novosis Putty has been designated as a breakthrough device by the US Food and Drug Administration (FDA).
This marks the first case of an implantable medical device developed by a South Korean company receiving FDA breakthrough device designation.
FDA's breakthrough device designation is a program designed to expedite the entry of groundbreaking medical technologies into the market. It provides benefits such as priority in the US approval process, flexible clinical trial design, and close support from a dedicated review team.
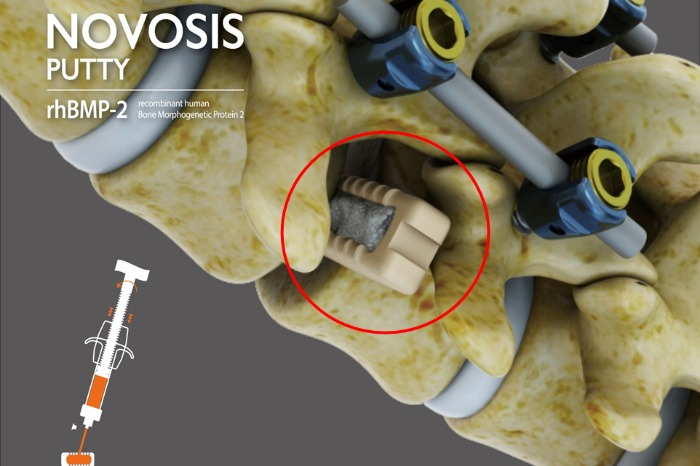
Novosis Putty is a bone substitute containing recombinant human bone morphogenetic protein (rhBMP-2). When bones are damaged, it aids in the differentiation of stem cells within the human body into bone cells, facilitating the formation of new bone.
CGBio plans to apply for US confirmation clinical trials to demonstrate the safety and efficacy of Novosis Putty in the first half of this year.
Write to Yoo-Rim Kim at
youforest@hankyung.com