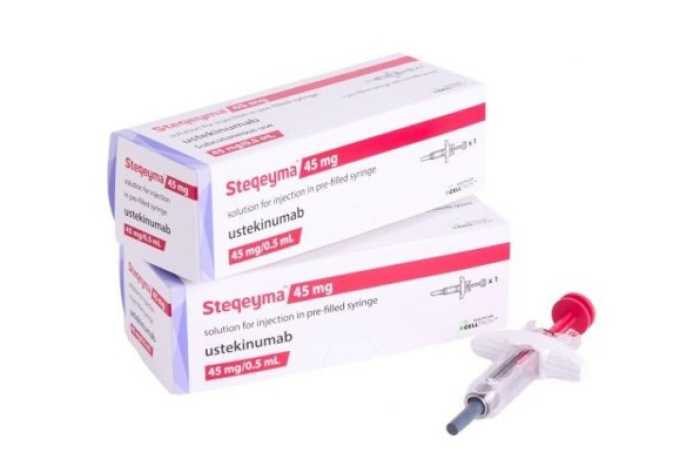
South Korea’s Celltrion Inc., received new drug submission (NDS) approval from Health Canada for Steqeyma, a biosimilar of the autoimmune disease treatment Stelara.
With this approval, Celltrion announced on Wednesday that it can now sell Steqeyma in Canada for the treatment of plaque psoriasis, psoriatic arthritis, and Crohn’s disease.
Celltrion plans to enter the North American market, the world's largest pharmaceutical market, starting with Canada, aiming to establish a strong presence in the global ustekinumab (the active ingredient in Stelara) market.
According to pharmaceutical market research firm IQVIA, the global ustekinumab market reached $20.4 billion last year.
The Canadian market size is around $663 million, but the total North American market, including the United States, is $16.4 billion, accounting for over 80% of the global market.
Celltrion received approval for Steqeyma in South Korea in June and subsequently received a positive opinion recommendation from the European Medicines Agency (EMA)’s Committee for Medicinal Products for Human Use (CHMP).
Once it obtains final European market authorization, the company’s efforts to penetrate the global ustekinumab market are expected to accelerate.
The company expects not only to expand its autoimmune disease portfolio in the North American market but also to strengthen its market position.
In addition to its existing tumor necrosis factor (TNF)-alpha inhibitor products such as Remsima, Remsima SC, and Yuflyma the expansion to include interleukin (IL) inhibitor products will broaden the range of patients it can serve.
Write to Jeong Min Nam at
peux@hankyung.com