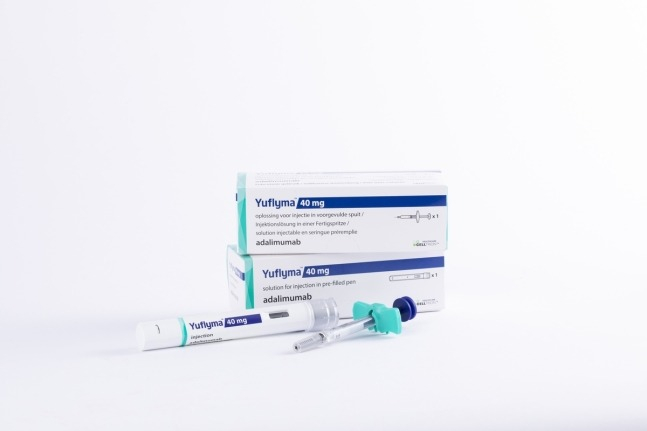
South Korea's Celltrion Inc. announced on Tuesday that its autoimmune disease treatment Yuflyma (Low WAC, active ingredient adalimumab) is now available for purchase at Costco in the US.
According to Celltrion, Yuflyma was registered earlier this month under the Costco Membership Prescription Program (CMPP) at a low wholesale acquisition cost (WAC).
CMPP is a program that allows Costco members to purchase medications at discounted prices at in-store pharmacies or affiliated pharmacies.
Starting this month, uninsured Costco members and their dependents can purchase Yuflyma at a discounted price in Costco stores and affiliated pharmacies across the United States at a reduced wholesale price.
The National Center for Health Statistics of the US Centers for Disease Control and Prevention (CDC) reported that the number of uninsured individuals in the US reached 27 million in the first quarter of 2024.
Write to Jeong Min Nam at
peux@hankyung.com