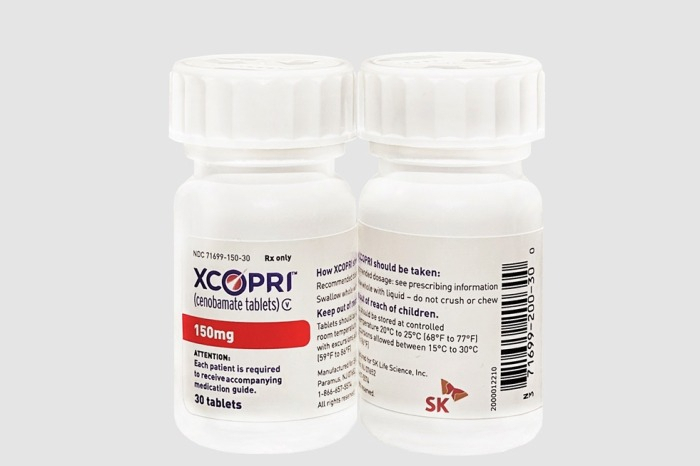
SK Biopharm submits NDA of epilepsy drug to China
The company got the $15 mn milestone payment after handing in NDA to China's NMPA
Dae-Kyu Ahn
1
2024-12-05 10:36:08
powerzanic@hankyung.com
Bio & Pharma
South Korea's SK Biopharmaceuticals Co. said on Tuesday it submitted the new drug application (NDA) for its flagship epilepsy drug Cenobamate to China's National Medical Products Administration (NMPA) through Ignis Therapeutics, the joint venture of SK Biopharmaceuticals and 6D Capital.
The company won the $15 mn milestone payment after handing in the NDA to NMPA.
China is the world's largest market for epilepsy drugs. In China, there are around 10 million people with epilepsy.
SK Biopharmaceuticals' Cenobamate has entered the US and European markets. The company also aims to expand into Greater China.
Write to Dae-Kyu Ahn at powerzanic@hankyung.com