Celltrion unveils cancer medicine to expand new drug pipelines

South Korea’s leading biosimilar drug developer Celltrion Inc. on Sunday unveiled an anticancer medicine to expand new pipelines and accelerat...

South Korea’s leading biosimilar drug developer Celltrion Inc. on Sunday unveiled an anticancer medicine to expand new pipelines and accelerat...
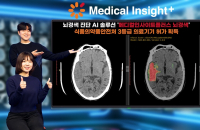
SK Inc C&C (SK C&C), the information technology service unit of South Korea’s SK Group, said on Thursday it has secured medical device...

South Korean biopharmaceutical company Celltrion Inc. announced on Wednesday that it has applied for CT-P39, a biosimilar of the treatment of asth...

South Korean biopharmaceutical company Celltrion Inc. announced on Friday that it applied for approval from the European Medicines Agency (EMA) for ...

South Korean biopharmaceutical company Celltrion Inc. announced on Friday that it has completed a patent agreement with Johnson & Johnson, the m...

South Korean biopharmaceutical company Celltrion Inc. announced on Tuesday that it has received approval from the European Medicines Agency (EMA) fo...

South Korea's biopharmaceutical company Celltrion Inc. announced that it received on Thursday approval from the US Food and Drug Administration (FDA...

South Korean biosimilar giant Celltrion Inc. revealed on Wednesday the highly anticipated clinical phase 1 data for its Actemra biosimilar, CT-P47, ...
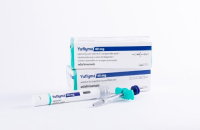
South Korean biopharmaceutical company Celltrion Inc. announced on Wednesday that it has obtained product approval from the US Food and Drug Adminis...

South Korea’s Celltrion Inc. is speeding up global clinical trials of its biosimilar of the multiple sclerosis (MS) treatment Ocrevus (active ...

South Korean biopharmaceutical company Celltrion Inc. announced Friday that it won its first trial against New York-based biotech giant Regeneron Ph...