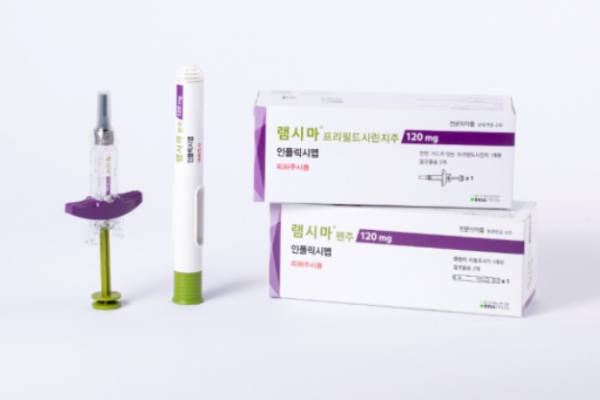
Celltrion Ltd. said on Friday it had applied the previous day for permission from the US Food and Drug Administration (FDA) for Remsima SC, an autoimmune disease treatment.
Remsima SC (infliximab) is a self-injectable drug developed as a subcutaneous injection formulation.
A Celltrion official said it applied to the FDA based on results confirming the efficacy and safety of the global phase 3 clinical trial for ulcerative colitis and Crohn's disease.
"The influence of Remsima SC is rapidly increasing with a market share of more than 12% in Europe, where it entered the market earlier, thanks to its rapid dosing effect and convenience of formulation," said a Celltrion official.
"Once it secures new drug status in the US, it will further strengthen the position of the Remsima family and provide more patients with high-quality drug treatment opportunities," he added.
Write to Jae-Young Han at
jyhan@hankyung.com