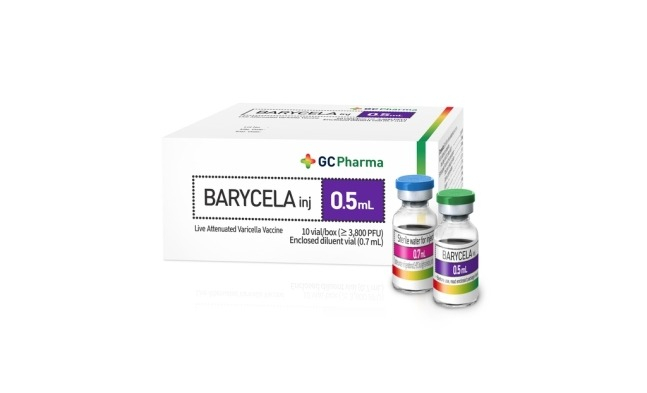
South Korea's GC Biopharma Corp. is set to regain the No. 1 spot in global production of chickenpox vaccines after earning qualification to enter the World Health Organization (WHO) procurement market with Barycela following its export hit Suduvax.
This breakthrough is expected to heat up global competition among domestic companies such as GC Biopharma and SK Bioscience Co. for such vaccines.
GC Biopharma on Monday said its chickenpox vaccine Barycela received WHO pre-qualification (PQ) certification, the fourth such vaccine worldwide to get approval after Merck of the US' Varivax in February 2018, SK Bioscience of South Korea's SKY Varicella in December 2019 and Sinovac Biotech of China's live attenuated varicella vaccine in November last year.
PQ certification allows a company to enter the procurement market for United Nations affiliates such as WHO, UNICEF and Unitaid, aka the International Drug Purchasing Facility. It is also a bidding qualification for the world's largest procurement market, the Pan American Health Organization (PAHO), which includes 35 countries like the US, Canada, Mexico and Brazil.
GC Biopharma, which released South Korea's first chickenpox vaccine Suduvax in 1993, ranked first on the global market for such vaccines until 2018. Global companies are shifting their product lineups toward more profitable and expensive vaccines, but GC Biopharma has expanded the market through price competitiveness and quality.
Scoring its highest chickenpox vaccine output of 61.5 billion won ($47.4 million) in 2018, the company saw performance plummet to 16.9 billion won the following year and a sharp drop in its global market. This was because of an output gap caused by changing of its product lineup to the new vaccine Varicella, which received approval to enter the market in 2020.
SK Bioscience, meanwhile, has rapidly expanded its market dominance since its vaccine SKY Varicella received WHO PQ certification in just a year after its launch in 2018. In February last year, its participation in bidding for PAHO resulted in a contract to supply chickenpox vaccines worth $31.27 million by 2024 on a market that GC Biopharma had topped in share in 2015, 2017 and 2019.
PAHO holds a bidding every two to three years and is expected to hold more tenders through late next year. GC Biopharma plans to expand the market based on its network.
Write to Ji-Hyun Lee at
bluesky@hankyung.com