South Korean cancer molecular diagnostics company Gencurix Inc. announced on Monday that it joins US President Joe Biden's cancer-conquering project "Cancer Moonshot."
The Cancer Moonshot is a policy by the Biden Administration aiming to reduce the cancer mortality rate by over 50% in the next 25 years. An annual investment of $1.8 billion is dedicated to introducing cancer treatments and diagnostic technologies.
To promote the Cancer Moonshot policy, the Biden government established a public-private partnership called CancerX in February this year, including participation from global pharmaceutical giants like Johnson & Johnson and AstraZeneca and global tech companies like Intel and Amazon.
Gencurix is also becoming a member of this CancerX initiative, marking the first case among domestic cancer molecular diagnostics companies. The company was recognized for its essential companion diagnostics technology in developing targeted cancer therapies, leading to its participation in the Cancer Moonshot project. Companion diagnostics refer to diagnostic methods that identify patients who would likely benefit from a specific drug before its use.
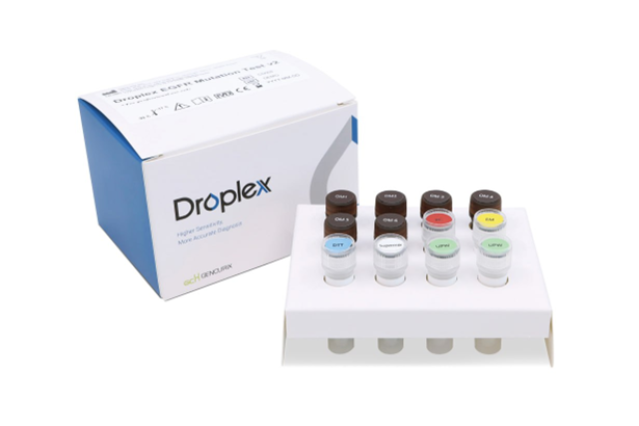
Gencurix has developed a companion diagnostic test called "Droplex," utilizing digital polymerase chain reaction (PCR) technology. It received European in-vitro diagnostic (IVD) medical device certification last year. The company explained that Droplex is a platform specialized in liquid biopsy, capable of detecting mutations with a small amount of DNA.
"We have secured an opportunity to collaborate with leading companies and research organizations in the cancer field and strengthen our global network. We will use this opportunity to showcase the technology of Droplex and further expand into the global market," the CEO of Gencurix Cho Sang-Rae said.
Write to Jeong Min Nam at
peux@hankyung.com