South Korea's medical AI company Lunit announced on Friday it will participate in the US President Joe Biden's cancer-conquering project Cancer Moonshot.
The company joins CancerX, a public-private partnership for Cancer Moonshot. CancerX introduced Lunit as the first case for cancer diagnosis among 14 participating companies, releasing Solution Catalog, the digital guide for cancer diagnosis and treatment in the US.
Solution Catalog is CancerX's first detailed project announcement since enlisting members for cancer eradication, focusing on digital innovation strategies to improve accessibility to cancer treatment and reduce financial burdens on patients.
CancerX divided the Solution Catalog into three areas: cancer diagnosis, treatment and care, and treatment management. It provides a guide for the commercialized digital products and solutions in each field for active utilization by US medical institutions.
As a result, medical institutions across the US now have immediate access to the digital cancer diagnosis and treatment products and solutions offered by the 14 companies introduced in the Solution Catalog.
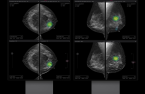
Lunit will concentrate on providing its Lunit Insight product line in the cancer diagnosis area of the Solution Catalog, contributing actively to the US government's Cancer Moonshot project through early diagnosis of lung and breast cancer.
"There is a statistic in the US that 4 out of 10 cancer patients spend all the money they have saved throughout their lives during cancer treatment," Lunit CEO Suh Beom-Seok said. He emphasized that CancerX's initiative, presenting the use of Lunit AI solutions for early cancer diagnosis by US medical institutions, is expected to ease the financial burden on patients and contribute to reducing the national healthcare budget.
Write to Young-Ae Lee at
0ae@hankyung.com