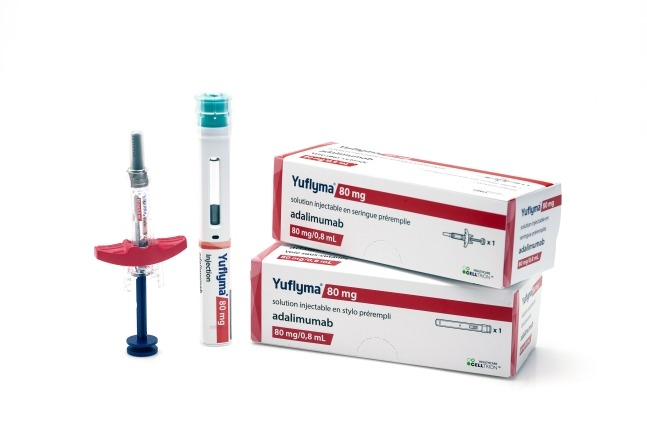
South Korea's Celltrion Inc. announced on Wednesday that it has applied to the US Food and Drug Administration (FDA) for a modified approval to replace its biosimilar Yuflyma with the original treatment Humira for the treatment of autoimmune diseases.
Humira is a blockbuster drug with global sales last year of $21.2 billion (27.4 trillion won), 87% or $18.6 billion of which came from the US. Available in autoinjector and prefilled syringe form, the treatment has secured indications in America for eight diseases including rheumatoid arthritis, Crohn’s disease, and ulcerative colitis.
Yuflyma, a biosimilar developed by Celltrion for Humira, obtained sales approval in the US last year.
If the biosimilar in the US receives approval for interchangeability with the original drug along with the previous product approval, it can be substituted at pharmacies without additional prescriptions from doctors, securing market competitiveness.
Consequently, many biosimilar developers seek clinical trials to confirm interchangeability with the original drugs in addition to clinical trials for approval.
Celltrion had previously demonstrated statistical equivalence between Yuflyma and Humira in a Phase 3 interchangeability clinical trial involving 367 patients with moderate to severe plaque psoriasis.
Celltrion expects that obtaining interchangeability recognition will have a positive impact on expanding Yuflyma's market share in the US.
Write to Jeong Min Nam at
peux@hankyung.com