South Korea's SK Biopharmaceuticals targeted 416 billion won ($311 million) for its epilepsy treatment Cenobamate in the US market this year.
SK Biopharm revealed this during the 2024 National Sales Meeting of its US subsidiary SK Life Science Inc., held in Tampa, Florida on Feb. 12 to 16.
Around 160 local employees, including SK Biopharm CEO Lee Dong Hoon, shared new goals and plans during this fifth annual sales meeting.
Cenobamate, known as Xcopri in the US, achieved sales of 270.8 billion won ($202.8 million) in the US market last year, representing a 60.1% increase compared to the previous year.
With a rapid increase in new patient prescriptions, the total number of prescriptions reached 26,000 in December last year.
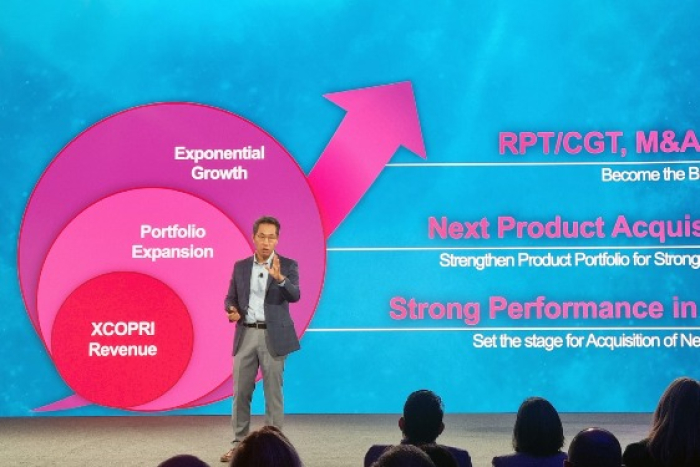
SK Biopharm aims to achieve an annual sales target of $311 million for Cenobamate in the US this year and plans to increase total prescriptions (TRx) to over 30,000, aiming to become the leader in the prescription drug sector.
"Building on the profitable performance of the last quarter, this year will be the first year that we achieve full-year profitability and demonstrate the profitability of our business model of direct drug sales in the US," Lee said.
"With the accelerated growth of Cenobamate, we can introduce a second product through the local sales network."
Write to Dae-Kyu Ahn at
powerzanic@hankyung.com