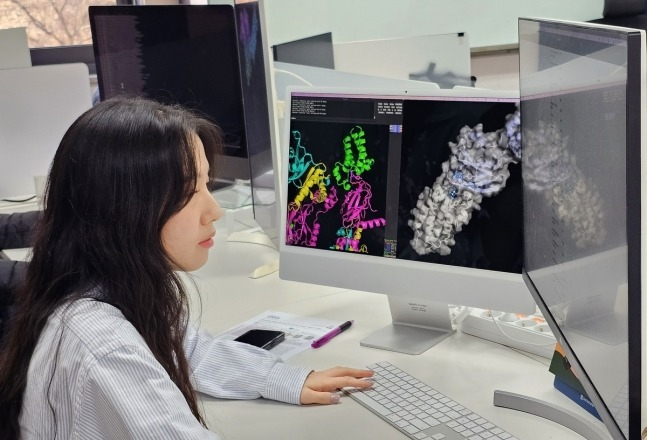
South Korea's Daewoong Pharmaceutical announced on Monday that it has established a drug development system Daewoong AI System (DAISY) which combines an 800 million-compound molecular model database with an artificial intelligence (AI) target substance discovery system.
According to the company, DAISY is an AI drug development portal that enables the discovery of compound substances and rapid prediction of drug properties.
DAISY incorporates the compound the substance database Daewoong Advanced Virtual Database (DAVID) and the tool for exploring drug candidates AI-based Virtual Screening (AIVS).
The globally available open-source compound substance data requires preprocessing to separate and remove unnecessary information. DAVID undergoes this process, creating a processed database of 800 million compounds that AI can utilize, explained Daewoong Pharmaceutical.
Similar to the biblical story where David defeated Goliath, DAVID signifies Daewoong's intention to compete head-on with global pharmaceutical companies in AI-driven drug development.
AIVS is a system based on 3D modeling that uses AI to discover active substances targeting specific proteins.
Daewoong reported a significant reduction in development time by utilizing the AI drug development system to discover active substances acting on two target proteins and applying them to the optimization phase.
The company plans to expand the use of AI drug development systems to various stages of drug development, including pre-clinical, clinical, and post-marketing phases.
Write to Ji-Hyun Lee at
bluesky@hankyung.com