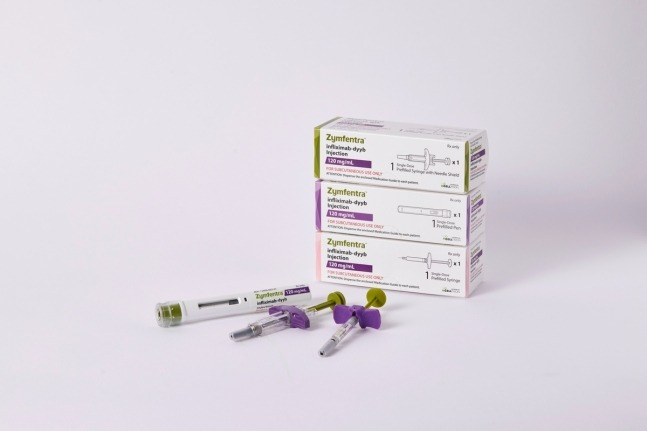
South Korea's Celltrion Inc. announced on Wednesday that it shipped the first supply of its subcutaneous injection type of the autoimmune disease treatment Remsima SC (active ingredient infliximab, American name Zymfentra) to the US.
The completed product shipped today will go through local logistics processes, including import clearance, transportation, and wholesale warehousing, and is expected to be available in the market from mid-March.
Celltrion plans to ship all three batches of the initial supply of Zymfentra from Wednesday until early March.
Zymfentra was developed by changing the existing intravenous injection type autoimmune disease treatment Remsima into a subcutaneous injection type, and it obtained new drug approval (NDA) from the US Food and Drug Administration (FDA) in October last year.
The product has obtained NDA in more than 50 countries worldwide, including Europe and Canada.
Write to Dae-Kyu Ahn at
powerzanic@hankyung.com