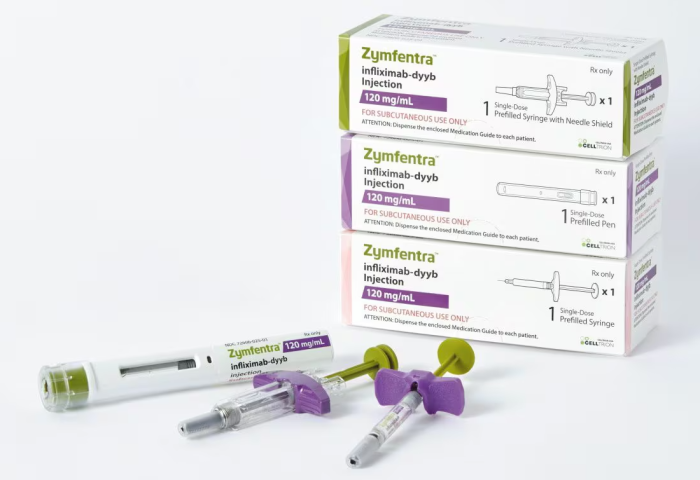
South Korean biosimilar maker Celltrion Inc. said on Monday that its subcutaneous injection formulation for autoimmune diseases, Zymfentra (infliximab), has joined the insurance-covered drug lists by three major US Prescription Benefit Managers (PBMs) after its US launch in March.
The three PBMs are Express Scripts, OptumRx and CVS Health. They control about 80% of prescription drugs covered by insurance in the US. They act as intermediaries between pharmacies, drug manufacturers and insurance companies.
The contracts were signed between April and early this month.
With one of the PBMs, Celltrion has secured a public insurance contract and is negotiating to get the biosimilar covered by private insurance plans as well.
Zymfentra, a self-administered version of Celltrion's intravenous biosimilar "Remsima," was approved as a new drug in the US last October.
It treats inflammatory bowel diseases like ulcerative colitis and Crohn's disease.
According to IQVIA, the inflammatory bowel disease treatment market in the US is estimated at 14 trillion won ($10.2 billion).
Celltrion aims to supply Zymfentra to 150,000 inflammatory bowel disease patients in the US by next year, or half of some 300,000 patients suffering from the disease in the country, according to Celltrion founder and Chairman Seo Jung-jin.
Write to Dae-Kyu Ahn at
powerzanic@hankyung.com