Daewoong wins permission for Nabota in Argentina

South Korea's Daewoong Pharmaceutical Co. announced on Monday that its botulinum toxin Nabota received product approval from Argentina's National Ad...

South Korea's Daewoong Pharmaceutical Co. announced on Monday that its botulinum toxin Nabota received product approval from Argentina's National Ad...

PharmaResearch Co., a South Korean company specializing in regenerative medicine, announced on Wednesday that it acquired TuringBio, an AI-based dig...

South Korea's Daewoong Pharmaceutical Co. announced on Thursday that it signed a contract with Indonesia's Bogor Agricultural University (Institut P...

South Korean biotech Xcell Therapeutics Inc., a developer of chemically defined media for in vitro cell culture, is aiming for a junior bourse Kosda...

Samsung Group announced on Tuesday that its Life Science Fund has invested in the US-based antibody-drug conjugates (ADC) and gene therapy technol...
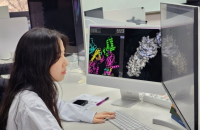
South Korea's Daewoong Pharmaceutical announced on Monday that it has established a drug development system Daewoong AI System (DAISY) which combine...

South Korea's Daewoong Pharmaceutical announced on Tuesday that its subsidiary Daewoong Biologics Indonesia has obtained a stem cell Lab Operational...

South Korea's Daewoong Pharmaceutical announced on Monday that it exported its technology for clinical development and commercialization rights of i...
Daewoong Pharmaceutical, a leading South Korean pharmaceutical company will export its new diabetes drug Envlo Tab. (active ingredient:enavogliflozi...

South Korea's Daewoong Pharmaceutical announced on Tuesday it signed with memorandum of understanding (MOU) with Merck Life Science to build an arti...

South Korea’s Daewoong Pharmaceutical on Wednesday said its gastroesophageal reflux (GER) disease drug Fexuclue (active ingredient fexuprazan ...

South Korea's Daewoong Pharmaceutical is venturing into the development of a product that can easily treat diabetic retinopathy in the form of ...
South Korea's Daewoong Pharmaceutical Co. announced on Thursday that it has received a patent from the US Patent and Trademark Office for the use of...

South Korea's Daewoong Pharmaceutical Co. announced on Friday that it has submitted an application for regulatory approval of its new diabetes treat...

South Korea's Daewoong Pharmaceutical announced on Tuesday that it has launched the GERD treatment drug "Fexuclue" (active ingredient: Fexuprazan) i...
South Korea's Daewoong Pharmaceutical said on Thursday that its local loyalty program for Nabota (American name: Jeuveau) in the US, a botulinum tox...

South Korea's biopharmaceutical company Celltrion Inc. announced on Monday that it has signed a contract with the US biotech firm Rani Therapeutics ...

South Korea's HanAll Biopharma Co. and Daewoong Pharmaceutical on Thursday said they concluded a contract with NurrOn Pharmaceuticals of the US on...

South Korea’s Daewoong Pharmaceutical said on Friday that it had concluded three technology export agreements for new drugs and new drug candi...
South Korea’s Daewoong Pharmaceutical next year will open a third plant to greatly expand annual output of its botulinum toxin Nabota at a cos...

South Korea's Daewoong Pharmaceutical announced on Friday that it has signed a technology export contract for the clinical development and commercia...
South Korea's Ministry of Food and Drug Safety announced on Wednesday that it has approved the cognitive therapy software "WELT-I" developed by We...

South Korean drugmaker Daewoong Pharmaceutical Co. said on Tuesday that it has signed a cooperation agreement with Sygnature Discovery, a British ne...

South Korean anti-cancer immunotherapy company Vaxcell-Bio Therapeutics announced on Friday that its natural killer (NK) cell therapy, Vax-NK/HCC, h...

South Korean pharmaceutical company Daewoong Pharmaceutical announced on Tuesday that it has received marketing authorization from Chile's National ...

South Korea's GC Biopharma Corp. announced on Thursday that it will start developing a flu vaccine based on messenger ribonucleic acid (mRNA).The dr...

South Korean drugmaker Daewoong Pharmaceutical Co. said on Tuesday it had launched its botulinum toxin product Nabota in Germany and Austria through i...

Daewoong Pharmaceutical Co., a leading South Korean pharmaceutical company, has announced the successful completion of phase 1 clinical trials for...

South Korea’s Daewoong Pharmaceutical Co. said on Feb. 24 it is set to embark on global sales of Envlo, a new drug for diabetes, by exportin...

South Korea’s health and drug safety authority has cleared Somzz to treat patients with sleep disorders, a dynamic move that is expected to vi...