S.Korea’s extra budget approved; IBs lift economic forecast

South Korea’s National Assembly, controlled by the ruling Democratic Party, on Friday passed the country’s new extra budget worth 31.8 t...

South Korea’s National Assembly, controlled by the ruling Democratic Party, on Friday passed the country’s new extra budget worth 31.8 t...
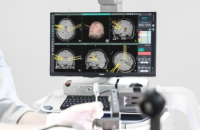
Koh Young Technology Inc., a South Korean developer of 3D optical inspection devices used in electronics manufacturing, is set to install its brain ...

Shares in KakaoBank Corp. soared as much as 17% to hit their highest level in nearly three years on Friday after the South Korean internet-only bank...

In a move that could reshape the global memory industry’s competitive dynamics, Nvidia Corp. has chosen US chipmaker Micron Technology Inc. as...

Woori Financial Group Inc., South Korea’s fourth-largest financial services provider, has received conditional approval from the country&rsquo...

Celltrion Inc. said Wednesday it has received regulatory approval from Australia’s Therapeutic Goods Administration (TGA) for three biosimilar...

Samsung Bioepis Co. and Celltrion Inc. are outpacing long-established global drug manufacturers such as Amgen Inc. and Sandoz in the antibody biosim...

Celltrion Inc.’s Avtozma, a biosimilar to Roche’s autoimmune disease treatment Actemra, has won approval from the European Commission, f...
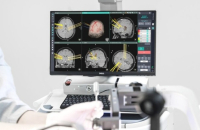
Koh Young Technology Inc., a South Korean developer of 3D optical inspection devices used in electronics manufacturing, said on Monday it got 501K...

KKR & Co. is facing a huge backlash from South Korean financial services firms after the debt restructuring of Dutch bicycle manufacturer Accell...
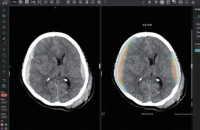
South Korea’s JLK Inc., an AI-based diagnosis solution and platform maker, said on Monday it got 501K approval from the US Food and Drug Admin...
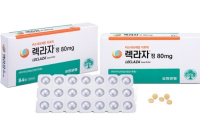
Leclaza (ingredient: lazertinib), a new lung cancer treatment developed by South Korea’s Yuhan Corp. secured approval from the European Commis...
South Korea's Celltrion Inc. said on Wednesday it received new drug submission (NDS) approval from the Food and Drug Administration (FDA) for Steq...

Celltrion Inc. is one step closer to commercializing its four new biosimilars in Europe after a committee of the European Medicines Agency (EMA) rec...

South Korea’s Celltrion Inc., received new drug submission (NDS) approval from Health Canada for Omlyclo, the biosimilar of allergic asthma an...

The European Commission (EC), the European Union (EU) antitrust body, approved the merger between Korean Air Lines Co. and Asiana Airlines Inc. on N...

South Korea's Noul Co., an in-vitro diagnostics maker and artificial intelligence (AI)-based blood and cancer diagnostic platforms company, said on ...

South Korea's Samsung Bioepis Co., Samsung Biologics Co.'s fully owned biosimilar subsidiary, and the US-based Biogen said on Tuesday the European C...

South Korea has designated Korea Zinc Inc.'s precursor manufacturing process as a national core technology and advanced industrial technology, effec...

South Korean chemical and food ingredient manufacturer Samyang Corp. said on Wednesday that it has become the first allulose producer to secure appr...

ViGenCell, a company specializing in immunotherapy, announced on Friday that it has obtained approval from the Ministry of Food and Drug Safety (MFD...
SK Bioscience Co., a bio and pharmaceutical affiliate of South Korea's SK Group, said on Wednesday that The Indonesian Food and Drug Authority BPOM ...

Celltrion Inc., South Korea’s largest biosimilar maker, said on Monday that it completed the establishment of a local subsidiary in Vietnam....

South Korea has approved the construction of two nuclear power plants, officially ending its anti-nuclear policy pursued by the previous Moon Jae-in...

SK Innovation Co., South Korea’s largest energy company, has won majority support from its shareholders for a merger with its liquefied natura...
A new lung cancer treatment developed by South Korea’s Yuhan Corp. has secured approval from the US Food and Drug Administration (FDA) as a co...

South Korea has decided to extend the range of missiles for export to 500 kilometers from the current 300 km after accepting requests to do so from ...

Celltrion Inc., South Korea’s biosimilar giant, will proceed with phase 3 trials for global blockbuster drug Keytruda’s copycat CT-P51, ...
South Korea’s Celltrion Inc., received new drug submission (NDS) approval from Health Canada for Steqeyma, a biosimilar of the autoimmune dise...

South Korean confectionery giant Lotte Wellfood Co. will merge its Indian subsidiaries — Lotte India Co. and Havmor Ice Cream Pvt — to b...