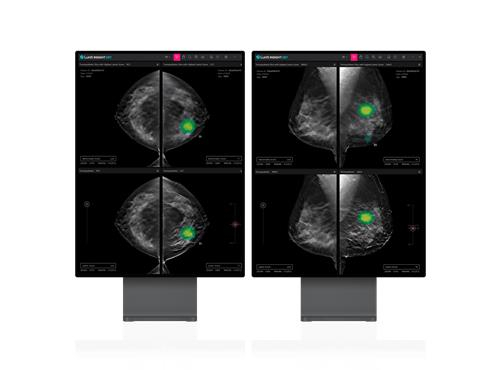
South Korean medical AI company Lunit said on Tuesday its Lunit Insight DBT, an AI imaging diagnosis solution aiding in breast cancer diagnosis, has secured 510(k) pre-market clearance from the US Food and Drug Administration (FDA).
This marks Lunit's third FDA approval, joining its AI emergency triage solution Lunit Insight Triage, and the breast imaging analysis tool Lunit Insight MMG.
The Lunit InsighT DBT analyses 3D images from digital breast tomosynthesis (DBT) with AI, offering faster and more accurate results than traditional 2D mammography.
With FDA approval, Lunit hopes to secure a share of the US market, which represents 65% of global breast cancer screenings, with DBT's widespread adoption.
"We will intensify our efforts in sales and marketing towards US healthcare providers, leveraging our precise AI solutions to swiftly grow our market footprint, responding to the substantial demand for accurate breast cancer diagnostics in the US," CEO of Lunit Suh Beom-Seok said.
Write to Young-Ae Lee at
0ae@hankyung.com