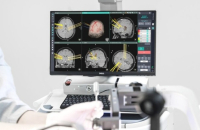
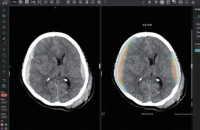
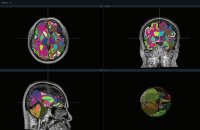
Allianz-backed Koh Young’s brain surgery robot set for US debut by end-August
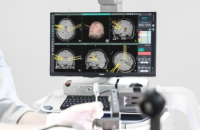
Koh Young Technology Inc., a South Korean developer of 3D optical inspection devices used in electronics manufacturing, is set to install its brain ...