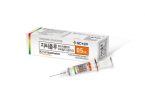
South Korea’s GC Biopharma Corp. announced on Monday that it received approval from the US Food and Drug Administration (FDA) for the immunoglobulin blood product Alyglo (intravenous immunoglobulin 10%.)
Alyglo is the treatment for primary humoral immunodeficiency. GC Biopharma submitted a biologics license application (BLA) for the drug to the FDA in 2021.
However, the COVID-19 pandemic disrupted plans, preventing a timely pre-license inspection of GC Pharma's blood product manufacturing facility in Ochang, North Chungcheong Province. This led to the FDA postponing the approval in February of the following year.
Subsequently, GC Biopharma underwent a pre-license inspection by the FDA at the Ochang facility in April. After further consultations, the BLA was resubmitted in July.
The company noted that this approval was received approximately one month before the initially set deadline of January 13, 2024, by the FDA.
GC Pharma also highlighted the growing U.S. immunoglobulin market, valued at approximately $10.4 billion (about 13 trillion won) last year.
The increasing demand for immunoglobulin in the U.S. is attributed to an aging population and a rise in autoimmune diseases, indicating a significant market opportunity for Alyglo.
Write to Ji-Hyun Lee at
bluesky@hankyung.com