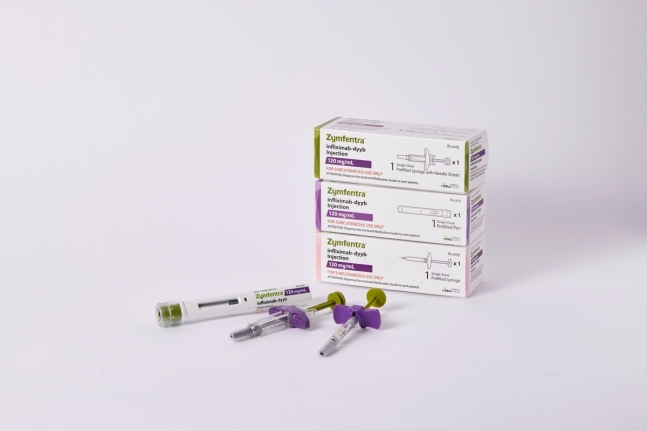
South Korea's Celltrion Inc. announced on Monday it received a new drug approval for an autoimmune disease treatment Remsima SC (active ingredient infliximab, American name Zymfentra) from the US Food and Drug Administration (FDA).
Zymfentra is Celltrion's first new drug approved by the FDA. The company filed a patent application related to the drug's formulation and mode of administration, aiming to protect its proprietary rights and maintain its patent until 2040.
Zymfentra is the subcutaneous injection (SC) version of the antibody biosimilar Remsima, which was previously developed by Celltrion. The drug has already been approved for sale in over 50 countries, including in Europe and Canada.
Celltrion shared that the FDA suggested the new drug licensing process right from the initial negotiation phases. Heeding this advice, Celltrion conducted two new global Phase 3 clinical trials and submitted the approval application in December last year.
The company projects that, post-launch, Zymfentra will yield an annual turnover exceeding 600 billion won ($443.7 million), with a three-year revenue forecast of 3 trillion won. Collaborative synergies are anticipated with previous US market entries Remsima and another autoimmune treatment Yuflyma, further solidifying their market presence in the US.
Zymfentra will be available in the US market via the direct sales network established by Celltrion Healthcare. Once the current merger is completed, Celltrion will emphasize the discontinuation of intermediary purchasing and selling activities by Celltrion Healthcare, which should enhance cost efficiency.
Write to Young-Ae Lee at
0ae@hankyung.com